Draw the Lewis Structure for Co32
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. -2 C- -2 0-C- o.
Lewis Structure For Co32 Carbonate Ion
Struktur Lewis Asam Karbonat.
. Lets learn step-by-step how to draw Lewis dot structure CO3 -2. A step-by-step explanation of how to draw the CO32- Lewis Structure Carbonate Ion. This video discusses the resonance structu.
Carbonic acid is a weak acid consisting two types of ions hydrogen ion H and carbonate ion CO32-. In this article carbonic acid lewis structure lewis structure drawing of carbonic acid and relevant detailed explanations are discussed briefly. Chemistry questions and answers.
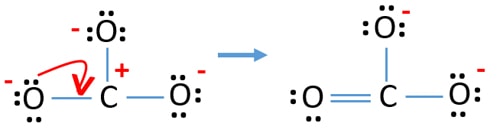
Carbonic acid is a weak acid consisting two types of ions hydrogen ion H and carbonate ion CO32-. We start with a valid Lewis structure and then follow these general rules- Resonance. The CO32-ion contains one C-0 single bond and two C-O double bonds.
According to him atoms achieve stable octet by gaining loosing or. Chemistry questions and answers. Solve Study Textbooks Guides.
Click hereto get an answer to your question Draw the Lewis structure for CO32 -. Lewis structure of CO3 2- ion Lewis dot structure of co3 2- lewis dot structure of co32-How to draw the Lewis Structure for CO3 2- Carbonate ionLew. State which orbitals or hybrids on C and O overlap to make each valence bond state each bond type s or p and the total bond order between each pair of bonded atoms.
Seeing the structure one would imagine that in carbonate ion two bonds will be the same in length the two single bonds and will be longer as compared to the third bond. Which of the following statements is TRUE. Group of answer choices Resonance structure contains one C-O single bond and two CO double bonds.
13 Draw the Lewis structure for CO32- including any valid resonance structures. Solution for Draw the Lewis dot structure for CO32- -2 -2 0. The Lewis structure for the carbonate ion is attached as shown.
Learn how to draw Lewis structures for ions carbonate ion electron dot structure of CO3 -2. Draw the Lewis structure for CO32 including any valid resonance structures. Asked Aug 2 2019 in Chemistry by GradStudent.
A carbonate is a salt of carbonic acid H2CO3characterized by the presence of the carbonate ion a polyatomic ion with the formula of CO3 2-. A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu. The CO32- ion contains two single bonds and one C_0 triple bond.
The lone pairs are the electron dots around the oxygen elements. And two single bonds between carbon and two negatively charged oxygen atoms. CO32- Lewis Structure - How to Draw the Lewis.
Resonance structure contains three CO double bonds. Lewis structure of carbonate ion is drawn in this tutorial step by step. The CO32- ion contains.
CO3 2- is carbonate. Using the formula the formal charge for each. Draw the Lewis structure for CO32- including any valid resonance structures.
The central Catom forms single bonds. Draw an octet-rule Lewis structure for CO32. Draw a Lewis structure for CO32- in which the central Catom obeys the octet rule and answer the following questions based on your drawing.
A The CO32- ion contains one C-O single bond and two CO double bonds. C The CO32- ion contains three C-O double bonds. Find total number of electrons of the valance shells of carbon and oxygen atoms.
There are equivalent three resonance structures CO32- the nitrite ion. The Carbonate ion is used frequently in chemistry and worth spending time to fully understand. In the CO32- Lewis structure carbon is the least electronnegative element.
Join Login Class 11 Chemistry Chemical Bonding and Molecular Structure Basics of Chemical Bonding Draw the Lewis structure for CO32 -. The CO 3 2- ion contains one C-O single bond and two C O double bonds. Gambar Dan Penjelasan Rinci.
Lewis Structure for CO 3 2- Carbonate ion. Describe one resonance structure of the carbonate ion. The number of unshared pairs lone pairs on the central Catom is.
Steps of drawing lewis structure of CO 3 2-Following steps are required to draw the CO 3 2-lewis structure and they are explained in detail in this tutorial. The following Lewis diagram represents the valence electron configuration. Which of the following statements is TRUE about one of the resonance structures.
Total valence electrons concept is used to draw the lewis structure of CO 3 2-After finishing the lewis structure of CO 3 2- there should be a -2 charge and it should be stabile structure. Draw the Lewis structure for CO32- including any valid resonance structures. B The CO32- ion contains two C-O single bonds and one CO double bond.
The formula for the formal charge is also given. Hydrogen carbon and oxygen have 1 6 and 8 electrons respectively. CO32- is an anion a negative ion seen frequently in chemistry.
The Lewis structure of the anion has one double bond between a neutral oxygen atom and carbon. In this article carbonic acid lewis structure lewis structure drawing of carbonic acid and relevant detailed explanations are discussed briefly. A simple notation used to represent valence electrons in an atom is called Lewis symbol.
For the CO32- Lewis structure the total number of valence electrons found on the periodic table for the CO32- molecule. Describe one resonance structure of the carbonate ion.
Lewis Structure For Co32 Carbonate Ion
Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

0 Response to "Draw the Lewis Structure for Co32"
Post a Comment